Preparation of pig organ samples to run on the NMR machine began with cutting and accurately weighing 4 grams of each organ: liver, heart and kidney.

Corinne cutting sections of kidney tissue

Lee-Anne carefully weighing 4 grams of tissue
The next step was to homogenise the sections in 10 ml of perchloric acid which blends the organ section and acid into a suspension together. After a demonstration by Dr. Bates, we continued with this until the organ sections were fully homogenised.

Corinne ensuring tissue sections are fully homogenised
This tissue/perchloric acid homogenates were then placed into a centrifuge for 10 minutes at 2,000 g (2,000 x the force of gravity) and the supernatant (containing the acid extractable metabolites) removed.

Lee-Anne placing samples into the centrifuge
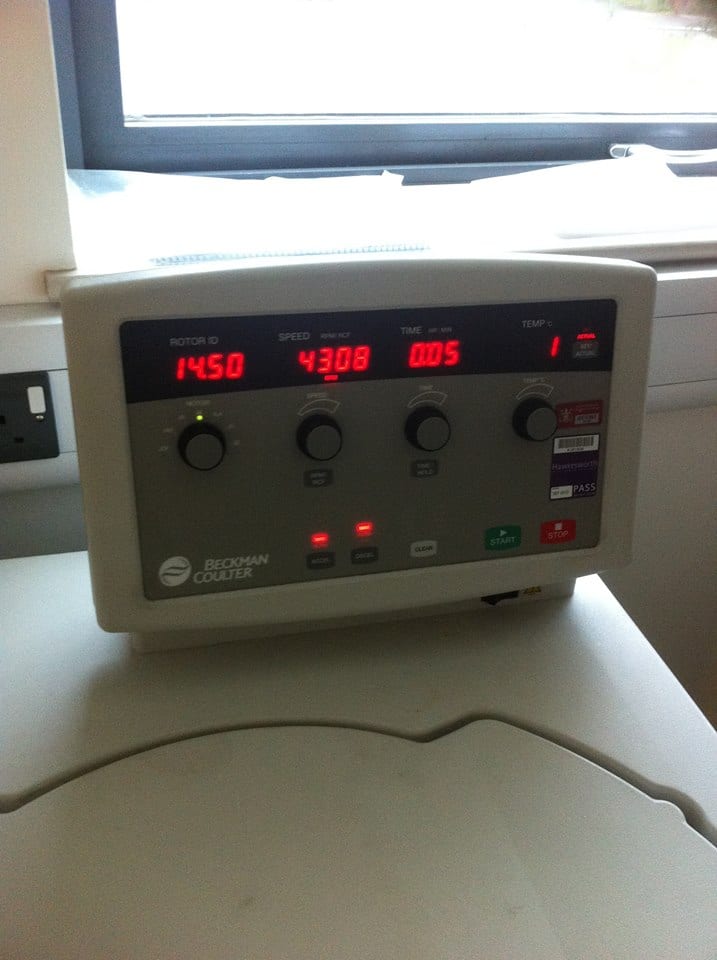
Samples were centrifuged
To neutralise this supernatant suspension to pH 7.00, 10 ml of sodium hydroxide was added. This was repeated for all samples until the desired pH was reached and a precipitate of potassium perchlorate was formed. The supernatant suspension was also placed in tubes in a centrifuge and centrifuges at 2,000 g (2,000 times the force of gravity) for 10 minutes to precipitate the potassium perchlorate.
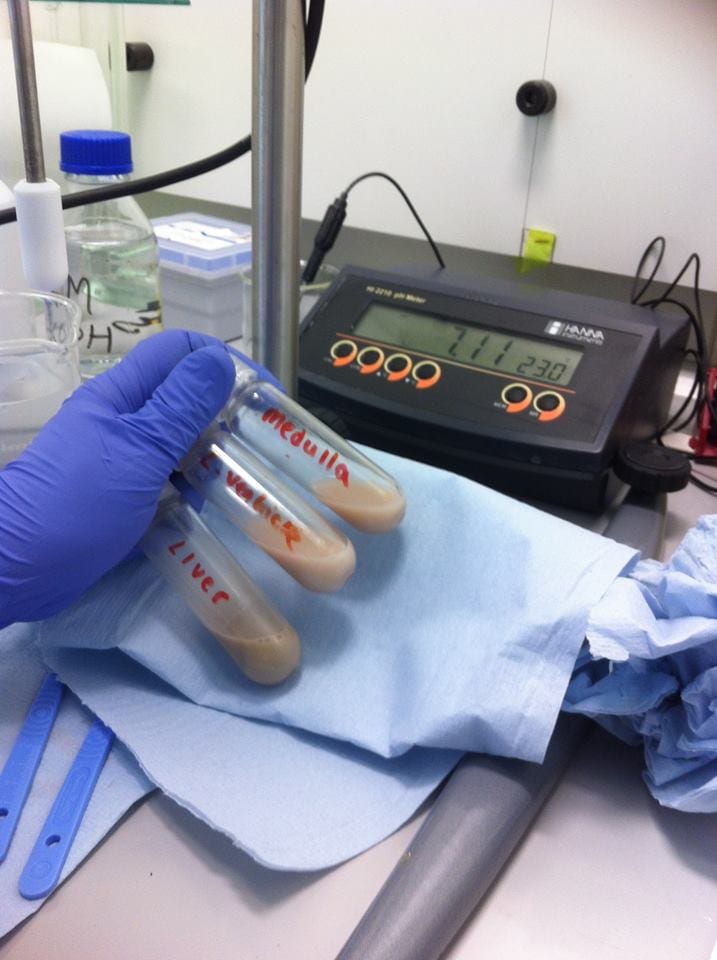
Samples were pH’ed and adjusted accordingly
The neutralised liquid tissue extracts were then pipetted onto a pre-equilibrated Chelex 100 chelating resin (layered into a fine glass Pasteur pipette) to remove any divalent cations, such as (Ferrous iron) Fe2+ or (Cupric copper) Cu2+, which can decrease the strength of the NMR signals obtained from biological tissue extracts, such as the ones we made.

Samples were extracted using Chelex100 to remove any divalent cation that might be present
3 ml of chelated sample were collected in small glass bottles and frozen in a -70 centigrade refrigerator. The glass bottles were then transferred into the freeze drying machine, and left overnight, to remove any water from the samples.