Dr. Prior firstly described the mechanisms and principles behind NMR spectroscopy; in that a very strong magnetic field, maintained by a large current (60 Amps) flowing around a circular magnet (supercooled by liquid nitrogen and liquid helium “jackets”), affects the polarity of nucleus (also known as protons) within the sample. This allows biological and chemical samples with different compositions to be identified and distinguished from each other. This is because nuclear spin gives them the properties of a magnet. However, he also explained that it is highly insensitive because only one in a million protons will align with the magnetic field generated by the NMR, rather than against it.
Following on from the preparation of the tissue extracts, and being shown how to operate the NMR spectrometer software, Dr Prior explained to us how to understand and interpret the proton (1H) NMR spectra.

Lee-Anne, Luke and Corinne studying the NMR
Dr. Prior teaching us individually how to analyse and interpret the NMR spectra.
Dr. Prior showed us the importance of the position of the protons on the y-axis and x-axis. These properties are used to help in substance identification. Furthermore, the fine structure of the peak is important in understanding the arrangement of protons. Dr Prior explained how analysing the chemical structure of a substance, particularly the positions of protons, can be used to predict the fine structure of peaks.

Dr Prior and Luke discussing chemical structure with regard to peak fine structure
Finally, we ran a two-dimensional NMR experiment on the sample of bile salts. This enables overlapping peaks on the spectra to be separated using the protons interaction with another atom.
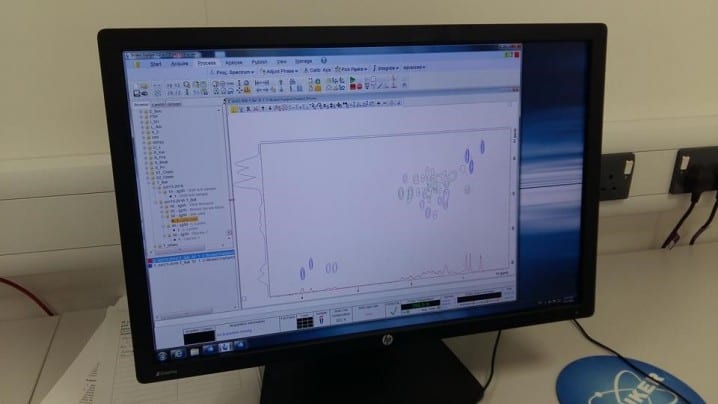
A two-dimensional spectra of bile salts obtained by the students using NMR

